The Persuasive G-CSF/PEG-G-CSF Market Report is a window into the industry that discusses market definition, classification, application, engagement, and market trends. Additionally, strategic models around growth objectives are designed by analysts, with detailed analysis of routes to market, competencies to be leveraged and developed, and any potential constraints. The G-CSF/PEG-G-CSF s Market Study also evaluates the market status, market share, growth rate, future trends, market drivers, opportunities and challenges, risks and entry barriers, sales channels, distributors and Porter's Five Forces Analysis. Leave no stone unturned when researching and analyzing data to prepare market research reports like this and others.
The large-scale G-CSF/PEG-G-CSF Market Report serves as an established source of information to offer a telescopic view of the current market trends, situations, opportunities and status. The report is divided into several characteristics which include manufacturers, regions, types, applications, market status, market share, growth rate, future trends, market drivers, opportunities, challenges, emerging trends, risks, entry barriers, sales channels, and distributors which are again detailed in the report as necessary to define the topic and provide maximum information for better decision making. Additionally, the excellent G-CSF/PEG-G-CSF Market report also provides a top to bottom assessment of the market with respect to revenue and emerging business sectors.
Data Bridge Market Research analyzes that the global G-CSF/ PEG-G-CSF market is expected to reach the value of USD 5,685.40 million by 2030, at a CAGR of 5.5% during the forecast period. This market report also covers pricing analysis, patent analysis, and technological advancements in depth.
Explore Further Details about This Research G-CSF/PEG-G-CSF Market Report https://www.databridgemarketresearch.com/reports/global-g-csf-peg-g-csf-market
.
| REPORT METRIC | DETAILS |
| Forecast Period | 2023 to 2030 |
| Base Year | 2022 |
| Historic Years | 2021 (Customizable to 2020-2015) |
| Quantitative Units | Revenue in USD Million, Pricing in USD |
| Segments Covered | By Indication (Neutropenia, Oncology, Chronic and Autoimmune Disease, Blood Disorders, Growth Harmony Deficiency and Others), Dosage (Mono and Combination), Route Of Administration (Intravenous, Subcutaneous), Packaging (Single Use Vials and Pre Filled Syringes), End User (Hospitals and Clinics, Research and Academic Institutes, Ambulatory Surgical Centers and Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy and Others) |
| Countries Covered | Mexico, Columbia, Brazil, Argentina, Chile, Peru, Ecuador, Rest of LATAM, Germany, U.K., France, Italy, Russia, Netherlands, Spain, Sweden, Poland, Belgium, Switzerland, Denmark, Norway, Finland, Turkey, Rest of Europe, China, Japan, India, Australia, New Zealand, Indonesia, Thailand, Vietnam, Singapore, Philippines, Malaysia, Rest of Asia-Pacific, South Africa, Saudi Arabia, Egypt, Israel, U.A.E, Kuwait, Oman, and Rest of Middle East and Africa |
| Market Players Covered | USV Private Limited, Viatris Inc., Biocon, Fresenius Kabi AG, Hangzhou Jiuyuan Gene Engineering Co., Ltd., Amgen Inc., Pfizer Inc., Sandoz International GmbH, Apotex Inc., Cadila Pharmaceuticals, Dr. Reddy’s Laboratories Ltd., Amneal Pharmaceuticals LLC., Coherus BioSciences, Accord Healthcare, NAPP PHARMACEUTICALS LIMITED., Intas Pharmaceuticals Ltd., Mundipharma International, Teva Pharmaceutical Industries Ltd., Spectrum Pharmaceuticals, Inc., Kyowa Kirin Co., Ltd., Jiangsu Hengrui Pharmaceuticals Co., Ltd., among others. |
Global G-CSF/ PEG-G-CSF Market Definition
Granulocyte colony-stimulating factor (G-CSF) is a medication used to treat neutropenia. This is a disease in which the number of white blood cells is lower than average and is caused by some forms of chemotherapy. The main types of G-CSF are lenograstim (Granocyte), filgrastim (Neupogen, zarzio, nivestim, accofil), long-acting (pegylated) filgrastim (pegfilgrastim, neulasta, pelmeg, ziextenco), and lipegfilgrastim (lonquex). Lenograstim is a glycosylated recombinant therapeutic agent that is chemically similar or identical to naturally occurring human granulocyte colony-stimulating factor (G-CSF). Various products include tablets and capsules and treat cancer, blood disorders, growth hormone deficiency, and chronic and autoimmune diseases.
Global G-CSF/ PEG-G-CSF Market Dynamics
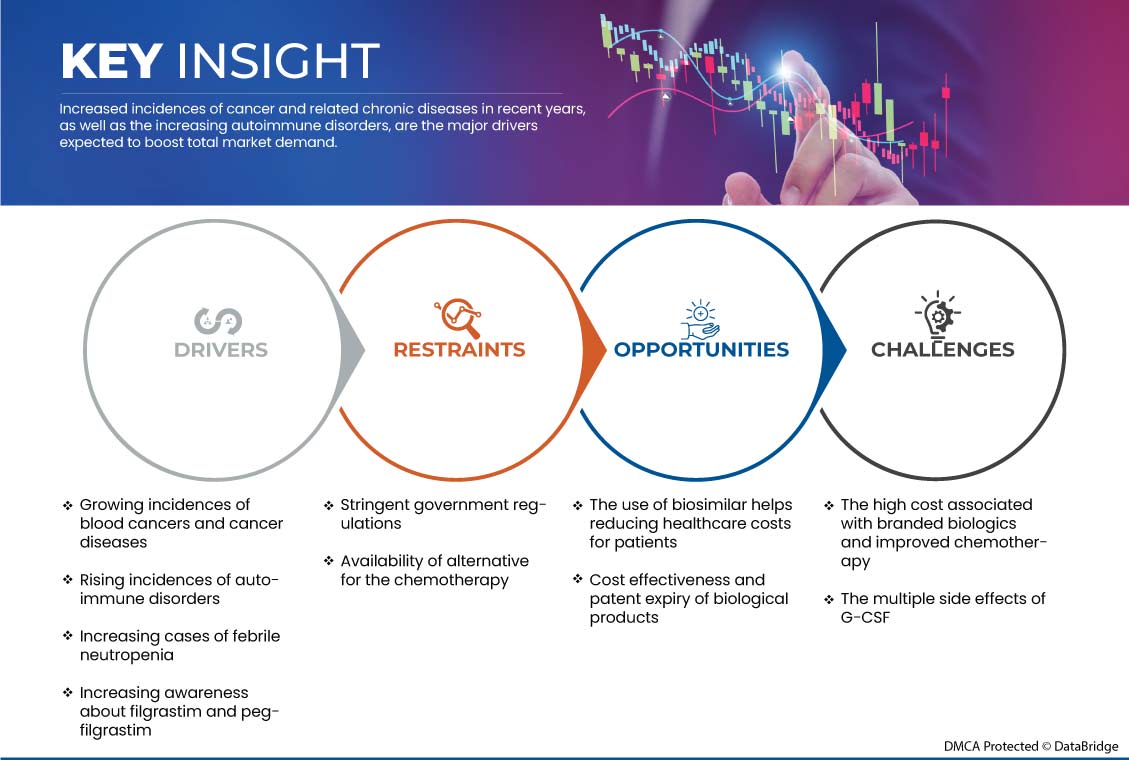
This section deals with understanding the market drivers, advantages, opportunities, restraints, and challenges. All of this is discussed in detail below:
Drivers
- Growing incidences of blood cancers and cancer diseases
Cancer is a general term for many diseases that can affect any part of the body. Other terms used for cancer are malignant tumors and neoplasm. One of the characteristics of cancer is the rapid formation of abnormal cells that grow beyond normal limits and can invade neighboring parts of the body and spread to other organs; the latter process is called metastasis. Extensive metastases are the leading cause of cancer death.
Filgrastim is a granulocyte colony-stimulating factor (GCSF) that helps increase the number of neutrophils in the blood. Filgrastim and pegfilgrastim are highly used to increase white blood cells after cancer chemotherapy or radiation therapy.
- Increasing cases of febrile neutropenia
Febrile neutropenia refers to fever during significant neutropenia. If a patient is neutropenic, their risk of infection may be higher than usual, and the severity of a particular infection may also be higher. Febrile neutropenia is the most common life-threatening complication of cancer treatment; its treatment is often an oncological emergency.
Febrile neutropenia is neutropenia accompanied by fever. Neutropenia refers to a decrease in the concentration of neutrophils in the blood. Neutrophils are a type of white blood cell that help fight infections as part of the immune system. The Infectious Diseases Society of America defines neutropenia as an absolute neutrophil count (ANC) of less than 1500 cells/mm3. The risk of infection and neutropenic fever increases dramatically with severe neutropenia, defined as an absolute neutrophil count (ANC) of less than 500 cells/mm3. Fever is defined as a single oral temperature greater than or equal to 101° Fahrenheit (38.3° Celsius) or a persistent temperature greater than or equal to 100.4° Fahrenheit (38.0° Celsius) or greater for one hour or longer.
Restraint
- Stringent Governmental Regulations
Pharmaceutical companies developing biosimilars such as filgrastim face a major challenge in the approval process for their products. Each country has a different approval process for all drugs, treatments, vaccines, and medical devices. However, these approval procedures are difficult to follow. This is due to the various regulations and evidence required to prove the effectiveness and safety of the product.
European Medicines Agency regulatory requirements ensure the same high quality, safety, and efficacy standards for biosimilars as for originator biologicals. They also include a rigorous comparability exercise with the reference product but are not universally accepted by regulatory bodies outside the European Union (EU). It should be noted that 'similar biologics' approved in India, 'biogenerics' approved in Iran, 'medicamento biológico similares' approved in Argentina, and non-originator biologicals approved in South Africa might not have been authorized if they had been subjected to the strict regulatory processes required for approval of biosimilars in the EU.
Opportunity
- The use of biosimilars helps reduce healthcare costs for patients
Biosimilars have the potential to fundamentally change healthcare by providing more affordable, equally effective treatments for patients and providing more treatment options for physicians. Developing biosimilars requires rigorous analysis to demonstrate their equivalence to the reference product and ensure no clinically meaningful differences in their safety, efficacy, and purity. As a result, health systems can channel long-term savings into overall improvements in patient care. To help create a thriving biosimilar market and ensure patient access, policymakers can take steps to reduce or eliminate the cost of biosimilars and encourage physicians to prescribe biosimilars compared to Europe.
Key Insights Of G-CSF/PEG-G-CSF Market Report
- The report comprises a detailed analysis of different segments and offers market valuations between 2023 to 2030.
- This study presents the analytical depiction of the G-CSF/PEG-G-CSF Market with the current trends and future estimations to determine the imminent investment pockets
- The report also reveals information with respect to key drivers, restraints, and opportunities coupled with a comprehensive analysis of the global G-CSF/PEG-G-CSF Market
- The forecast period of the G-CSF/PEG-G-CSF Market is assessed from 2024 to 2030 to highlight the G-CSF/PEG-G-CSF Market growth scenario.
- Porter’s five forces analysis establishes the effectiveness of the buyers and suppliers in the business line.
- The key players in the industry are profiled to gain an understanding of the strategies adopted by them.
- This report provides the current trends and future estimations during the forecast period, which in turn aids in identify the prevailing market opportunities.
The company portfolio includes company synopsis, operating business sectors, business overview, product/service categories, and recent growth strategies adopted by them.
Browse Related Reports:
Smart Air Conditioner Market by Size, Share, Forecasts, & Trends
Parametritis Treatment Market Size | Statistics Report, Share, Forecast, & Trends
Morton's Neuroma Treatment Market Size, Share & Trends Analysis Report
Hospital Mobile X-ray Market Size and Forecasts, Share and Trends
Liquid Malt Extracts Market Size, Industry Share, Forecast
Asia-Pacific Marine Ingredients Market | Size,Share, Growth
North America Color Management and RIP (Raster Image Processors) Software For Digital Textile Printing Market Size, Trends & Growth Analysis
About Data Bridge Market Research:
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email – corporatesales@databridgemarketresearch.com