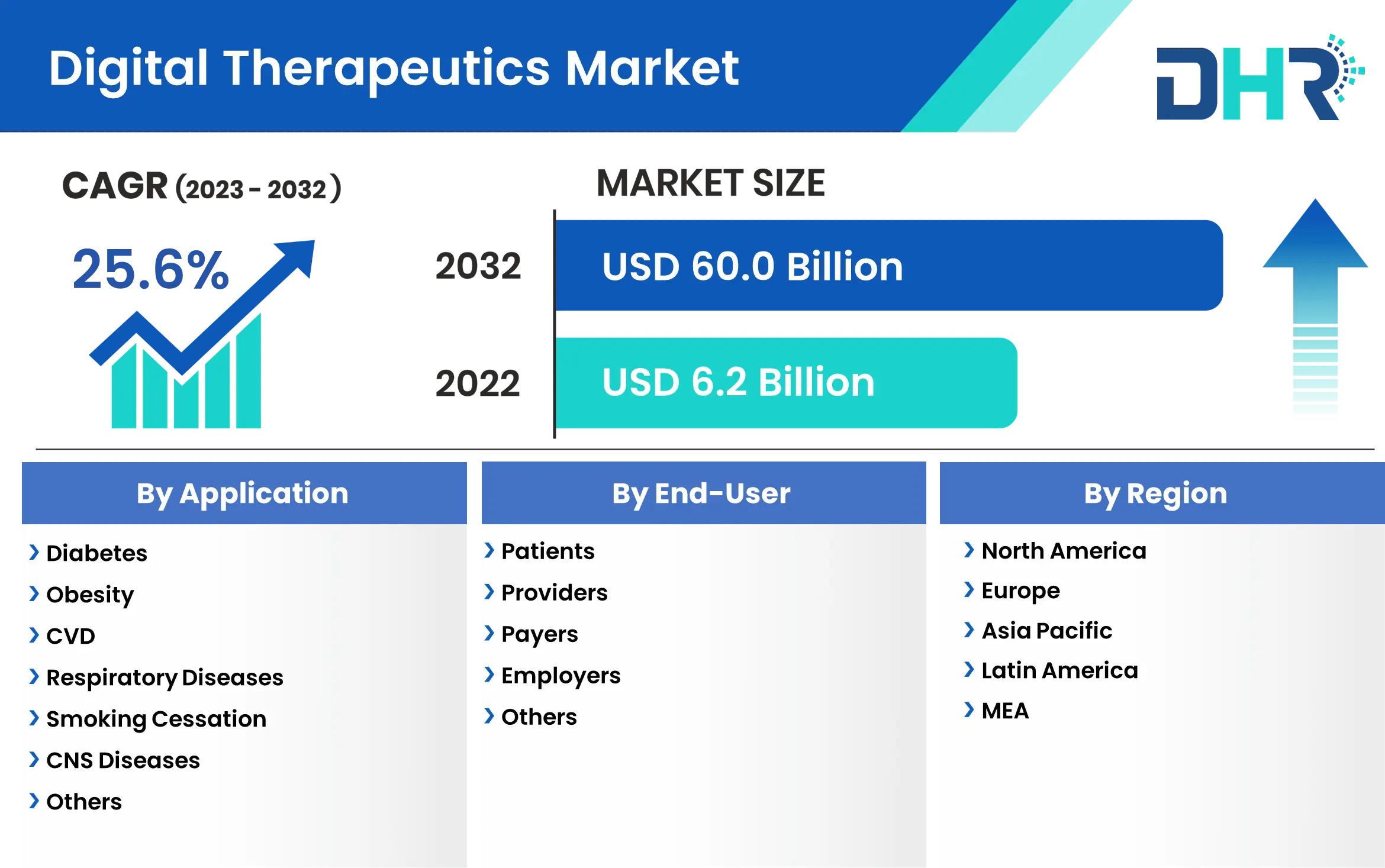
The digital therapeutics market size was valued at USD 6.2 Billion in 2022 and is expected to reach a market size of USD 60.0 Billion by 2032 with a CAGR of 25.6%.
The Digital Therapeutics Market encompasses a rapidly evolving sector within the healthcare industry, offering innovative solutions that leverage digital technologies to prevent, manage, or treat various medical conditions. Digital therapeutics, also known as software-as-a-medical-device (SaMD), deliver evidence-based interventions through software programs or mobile applications, often complementing or even replacing traditional medical treatments. These digital interventions are designed to address a wide range of health issues, including chronic diseases like diabetes, hypertension, and obesity, as well as mental health conditions such as depression, anxiety, and substance abuse disorders.
Request Sample Report: https://datahorizzonresearch.com/request-sample-pdf/digital-therapeutics-market-2292
The growth of the Digital Therapeutics Market is driven by several key factors:
- Rising prevalence of chronic diseases: The increasing prevalence of chronic conditions such as diabetes, cardiovascular diseases, and mental health disorders has created a significant demand for innovative and effective treatment solutions.
- Advancements in technology: Rapid advancements in technology, particularly in mobile computing, wearable devices, artificial intelligence, and data analytics, have paved the way for the development of sophisticated digital health solutions.
- Shift towards value-based care: Healthcare systems worldwide are increasingly transitioning towards value-based care models, which prioritize the delivery of high-quality, cost-effective healthcare services.
- Growing acceptance and adoption of digital health solutions: There has been a gradual shift in attitudes towards digital health solutions among healthcare providers, payers, regulators, and patients.
- Regulatory support and validation: Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established frameworks to evaluate and approve digital therapeutics as medical devices.
- Integration with traditional healthcare: Digital therapeutics are increasingly being integrated into traditional healthcare delivery models, including primary care, specialty care, and chronic disease management programs.
Segmentation:
By Application
- Diabetes
- Obesity
- CVD
- Respiratory Diseases
- Smoking Cessation,
- CNS Diseases
- Others
By End-user
- Patients
- Providers
- Payers
- Employers
- Others
Regional Analysis:
North America, especially the United States, leads the way in the adoption of digital therapeutics. The region benefits from a mature healthcare system, advancements in technology, and a supportive regulatory landscape, all of which have contributed to the expansion of digital therapeutics. Initiatives such as the FDA's Digital Health Innovation Action Plan and the establishment of regulatory pathways for software as medical devices have spurred innovation and facilitated market entry for digital therapeutics companies. Furthermore, collaborations among healthcare providers, payers, and technology firms have accelerated the adoption of digital therapeutics across the region.
In Europe, there has been notable progress in the uptake of digital therapeutics. The region boasts a well-established healthcare infrastructure and a growing emphasis on digital health initiatives. Regulatory bodies like the European Medicines Agency (EMA) and the Medicines and Healthcare Products Regulatory Agency (MHRA) have devised frameworks and guidelines for evaluating and approving digital therapeutics. Key markets such as Germany, France, and the United Kingdom have emerged, with reimbursement policies in place for specific interventions. Collaborations between digital therapeutics firms and healthcare stakeholders in Europe aim to drive implementation and generate evidence supporting the effectiveness of these interventions.
Key highlights of the report include:
- The report provides an analysis of the current market size of the digital therapeutics sector and forecasts its growth trajectory over the forecast period.
- It identifies and analyzes key trends driving growth in the digital therapeutics market, such as increasing prevalence of chronic diseases, advancements in technology, shifting healthcare delivery models, and regulatory support.
- The report offers insights into the competitive landscape of the digital therapeutics market, including profiles of major players, their market share, key strategies, and recent developments.
- It provides an overview of the therapeutic areas targeted by digital therapeutics solutions, including chronic diseases like diabetes, cardiovascular conditions, respiratory disorders, mental health conditions, and substance use disorders.
- The report discusses the regulatory environment governing digital therapeutics, including key regulations and guidelines issued by regulatory agencies such as the FDA, EMA, and others.
Contact:
DataHorizzon Research
North Mason Street, Fort Collins,
Colorado, United States
Ph: +1-970-672-0390
Website: https://datahorizzonresearch.com/
Email: sales@datahorizzonresearch.com